Technology
Technology
Using the BiCHEM technology, chemicals or biofuels can be produced from biomass. The three major constituents of biomass are hemicellulose, cellulose and lignin. BiCHEM aims to valorize the (hemi-) cellulose portion of the biomass.
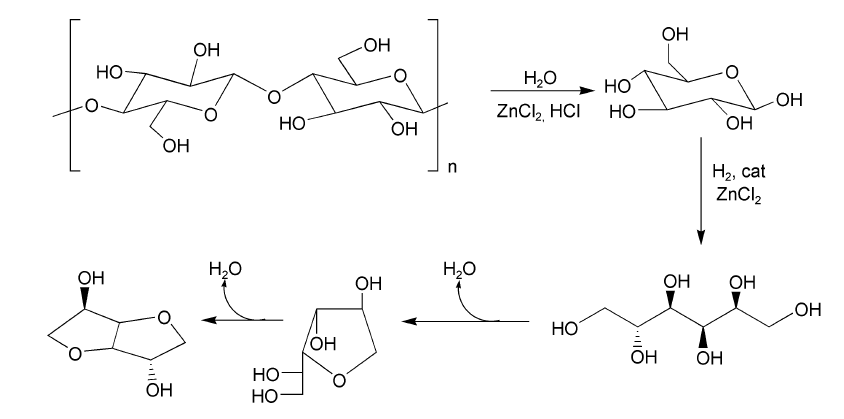
The first challenge of the process is to dissolve the biopolymers hemicellulose and cellulose and depolymerize them by a hydrolysis reaction to sugar monomers. Several methods are known to achieve this and a distinction can be made between acid and enzymatic hydrolysis, both with their advantages and disadvantages. In case of enzymatic hydrolysis, reaction rates are rather low, while yields of sugar monomers are high. In the case of acid hydrolysis (e.g. using sulfuric acid, a mineral acid) hydrolysis rates are high, but degradation processes occur simultaneously, compromising the yields. The BiCHEM technology is based on the use of acid hydrolysis to dissolve the (hemi-) cellulose. However, by using a molten salt hydrate (zinc chloride) instead of the commonly used mineral acids, fast dissolution and hydrolysis is combined with very high yields.
The next step in the process is to convert the sugar monomers (mainly glucose and xylose). These molecules are fairly unstable and are therefore first hydrogenated to respectively sorbitol and xylitol. This improves the product stability and keeps the overall process yields high. From sorbitol the very interesting platform chemical isosorbide can be made by a two-step dehydration. Isosorbide is a diol that can be used in the polymer industry (e.g. as a substituent for bis-phenol-A), but it can also be used as a starting point to synthesize for example the green solvent di-methyl-isosorbide or can be used to synthesize fuel additives.
Since the sorbitol dehydration step can also be catalyzed by the zinc chloride hydrate solvent, the whole process from biomass to chemicals can be carried out in the same solvent, as shown schematically in the Figure 1 below. An additional advantage of this scheme is that during the different chemical conversions the polarity of the product is reduced which makes separation of the final product form the polar molten salt hydrate solution easier.
Figure 1. Hydrolysis of cellulose to D-glucose, hydrogenation to glucitol, dehydration to sorbitan (1,4-anhydro-glucitol pictured), and sequential dehydration to isosorbide. See also: Almeida et al., ChemSusChem, 3(3),2010, 325-328.